The Need
The average life expectancy in the U.S. barely increased this year, which alarmingly represents the first time it has even increased at all in four years. This is unlike some other highly industrialized countries in which life expectancy has been increasing steadily over the past four (and more) years. While there are numerous reasons for this, one is the extremely high barrier that exists for patients to even know they have a healthcare problem and, upon such a realization, make an appointment to see a doctor [1]. It is clear that we need to make healthcare more accessible, and bring the hospital to the patient, in order to reverse these disheartening trends in life expectancy.
The poor increase in life expectancy in the U.S. compared to the rest of the world is also indicative of a healthcare system that generalizes treatments instead of making them personal for that specific patient, relies too heavily on pharmaceutical agents to “fix” everything, is “reactive” rather than “proactive”, and does not empower the patient to return to an active lifestyle after an injury. This is true for members of the military also, where a lack of empowerment after an injury often leads to a loss of motor activity, lack of feeling of contributing to the unit’s mission after a significant injury, depression, and sometimes even a loss of life. As just one of many examples, consider a soldier with an orthopedic injury suffered on the battlefield which leads to insertion of an implant, recovery, and physical therapy. Alarmingly, soldiers suffering from a bone fracture will receive the same implant an elderly woman receives when falling down the stairs, yet, their needs and reason for an orthopedic implant are much different. If civilians and soldiers had more control over their health, for example, to both monitor possible disease or bone fractures, earlier intervention of healthcare problems would be possible which would clearly lead to people more engaged in their health and better healthcare outcomes. Think, for example, of a soldier on the battlefield who is able to detect a small hairline bone fracture (before pain), fix that fracture, and quickly return to their duties. This not only obviously aids in their physical abilities, but mentally, returns confidence, a feeling of contribution, and lifetime of service to our country. It would be a revolution in military medicine.
Nanomedicine, or the use of materials with nanometer dimensions, may provide the answer to all that ails our current impersonal healthcare system. Specifically, implantable nanosensors can essentially bring the hospital to inside a patient’s body to better prevent, diagnose, and even treat a disease – all in one. The Defense Advanced Research Projects Agency has an extensive program to design and evaluate the use of implantable nanosensors that can detect healthcare problems before they even become healthcare problems [2]. While the idea is exciting and is destined to improve the quality and quantity of life for all who receive such an implantable nanosensor, developing implantable nanosensors is quite complex. One needs body-friendly materials, a sensor component, a method of communication, and, one that most people forget, a response mechanism to change adverse healthcare events after they are detected. It is also clear that implantable sensors involve many fields requiring not only strong technical expertise but also considerations for data security, data storage, and other factors involved when real time healthcare data can be collected every second of every day. Imagine your neighbor logging into your nanosensor to monitor (and control) your health! Despite these challenges, if we want to improve our life expectancy and quality of life as we age, we need to move forward and create such implantable nanosensors, for which are already so close.
The Idea
So, let me describe my study team’s idea to fabricate and use an implantable nanosensor to improve medicine. While this example was tailored for orthopedics (specifically, hip implants), the idea is translatable to all of medicine. Back when developed, hip implants revolutionized medicine, but few advances have been made in their design and use since then and they represent a part of our healthcare system that “reacts” rather than “predicts” healthcare problems. For this project, in order to increase our chances for implementation into medicine, we started by buying off-the-shelf titanium-based implants. (By using current medical devices, we could provide for an easier transition into implantable nanosensors). We then followed an anodization process to create nanopores into the titanium implant. Then, using chemical vapor deposition (without toxic catalysts), we grew carbon nanotubes out of the now nanoporous titanium implant. First, nanopores in titanium were necessary in order to securely fix the carbon nanotubes into the titanium implant. Orthopedic implant surgeries are not gentle. Mechanical tests have shown that when using typical orthopedic surgical equipment, the carbon nanotubes only stayed in place, fixed in the titanium, when they were grown out of the nanopores. Secondly, carbon nanotubes are the sensing component of our sensor. As has been well established, carbon nanotubes are electrically active and can be used to measure electrical properties of the cells that attach and the tissue that has grown on the implant. Since osteoblasts, bacteria, and scar tissue forming cells have different electrical properties, it is the carbon nanotubes that enable our sensor to work. Specifically, we use cyclic voltammetry to determine cellular and tissue forming events around the implant once in the body.
Further, we have incorporated radio frequency technology (such as that currently used in pace-makers) for sensor communication to a hand held device. Lastly, we have created a new polymer, a combined form of poly-lactic acid and polypyrrole, which can be electrically activated to degrade to then release a drug (for example, and antibiotic, anti-inflammatory, or bone growth factor) necessary to ensure implant success. The polymer is incorporated onto the sensor surface yet still allows for the carbon nanotubes to protrude in order to assess cellular events. The sensor is picture in figure 1.
The Performance
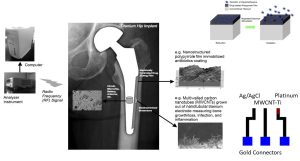
Of course, one critical question for any implantable sensor used in medicine is whether the sensor is biocompatible (of which many are not) and can it still sense biological events once in the body. We have published numerous in vitro studies providing evidence that not only is this sensor biocompatible, but it actually promotes bone growth more than current titanium-based implants (even without sensing or responding to biological events) [3-7]. However, the proof always comes through in vivo studies.
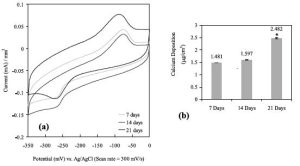
Thus, we implanted the sensor (as mentioned above, with the polymer coating embedded with gentamicin, an antibiotic, in some portions and bone-morphogenic protein-7, a known bone forming agent, in other portions) into the calvaria of rats for up to 7 days and used our sensor as well as typical histological and push out test to assess device performance. We also pre-seeded the implant with 105 CFU of Staph epidermidis, a bacterium which commonly infects implants. First, we used cyclic voltammetry to determine what biological events were occurring. Initially, we saw reduction-oxidation peaks indicative of bacteria and released gentamicin from the polymer coating after 1 day which proved to kill the bacteria. We then released bone-morphogenic protein-7 to promote bone growth and quickly saw characteristic reduction-oxidation peaks indicative of bone which grew with time (Figure 2). This is in contract to the control titanium implant without a sensor which showed increase bacteria presence with time.
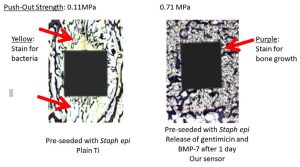
Traditional histology and push out tests matched the information we received from our sensor of greater bone growth and less bacteria on our implant with an embedded sensor compared to the control titanium without a sensor (Figure 3).
Now, imagine the real-world consequence of this implantable nanosensor for service members. Of course, bone fractures are a common healthcare problem for service members which may lead to implant surgery. Unfortunately, orthopedic implants fail too often due to infection and poor bone growth from a variety of reasons. But most importantly, few patients return to the active lifestyle they had before their catastrophic bone fracture. This type of implantable nanosensor can enable a service member to monitor, in real time, the success or failure of their implant. They can monitor the health of their bone next to an implant. They can observe whether bacteria have infiltrated the implant at pre-infection levels, and control the sensor to kill such bacteria and promote bone growth. This can be done anywhere and at any time to ensure a quick active return to the battlefield. And, this is just an example for orthopedics, think of the promise for any part of the body or healthcare problem that might exist.
The Future
Of course, such results imply an ability to detect implant failure well before what can be accomplished today with traditional X-rays, bone scans, or blood cultures for bacteria. By detecting implant failure earlier, success is likely especially in an approach like this in which biological events can be reversed without removing the implant. It is also hoped that over time, the sensor could determine if the implant separates from bone leading to failure. This is especially important for soldiers who are active on the battlefield and under high use. Such an implant is prone to additional fracture in the bone surrounding the implant. Further, while issues such as long-term power, data security, data storage, and others are still being explored, the present results of being able to detect cellular events around an orthopedic and change them on demand are quite promising to the future of healthcare.
Such sensors have also been incorporated into catheters, endotracheal tubes, vascular stents, neural probes, and others further expanding the role that implantable nanosensors will have in the future of medicine. In some of the most exciting future applications, such sensors are being incorporated into nanoparticles which can roam the body and send information concerning cell mutations, individual bacteria presence, the initial formation of blood clots and so much more at times and quantities much less than what is currently available with conventional methods. Think of the day when a soldier can monitor his or her own health, especially while deployed, rather than rely on a hospital. The possibilities are endless to improving healthcare. It is our hope that implantable nanosensors have a very bright future for military medicine, and also to hopefully one day reverse current poor increases in U.S. life expectancy.
REFERENCES
- Sara Heath, “Top Challenges Impacting Patient Access to Healthcare,” accessed at https://patientengagementhit.com/news/top-challenges-impacting-patient-access-to-healthcare, September 13 (2019).\
- The Defense Advanced Research Projects Agency, “In Vivo Nanoplatforms (IVN),” accessed at https://www.darpa.mil/program/in-vivo-nanoplatforms, February 5 (2020).
- S. Sirivisoot and T.J. Webster, “Multiwalled carbon nanotubes ce electrochemical properties of titanium to determine in situ bone formation,” Nanotechnology 19(29): 295101-295113 (2008).
- S. Sirivisoot and T.J. Webster, “Is my implant working: Nanotechnology sensors for determining bone growth,” BoneZone®Online Magazine, Strategic Sourcing for the Orthopaedic Industry (2007).
- S. Sirivisoot, R. A. Pareta, and T. J. Webster, “Electrically-controlled penicillin/streptomycin release from nanostructured polypyrrole coated on titanium for orthopedic implants,” Solid State Phenomena 151: 197-202 (2009).
- S. Sirivisoot, T.J. Webster, “Nanotechnology enabled in situ orthopaedic sensors for personalized medicine,” Biomedical Applications of Smart Technologies, 86: 40-50 (2013).
- S. Sirivisoot, T.J. Webster, “Nanotechnology-derived orthopedic implant sensors integrated microsystems,” International Journal of Nanomedicine 650-667 (2016).


