Background Optimal cognitive performance is critical to warfighter success. Methods to improve cognitive performance could enhance decision making, improve perception and even decrease deadly aviation accidents. [1,2] The Army, Air Force and Naval Research Laboratories are all actively researching new methods to improve warfighter cognitive performance. [3,4,5]
Sustained attention, in particular, is difficult for even the healthiest of individuals due to natural occurrences including circadian rhythms, fatigue, nutritional imbalances, etc. Shift work further deteriorates vigilance. This chronic disruption of circadian rhythms results in lower cognitive functioning in terms of processing speed, selective attention and memory. [6] Reaction time and errors of commission also worsen with shift work and long work hours. [7]
Even under optimal conditions, individuals absorb visual and auditory information with varying proficiencies. Processing both visual and auditory data simultaneously can be challenging even over short durations, particularly if rapid decision-making is required. This can significantly stress/impair performance, which typically declines over time. Distractibility often occurs as vigilance declines. It occurs when one becomes hypersensitive to immediate environmental disturbances. Whether brain resources are reallocated to block distractions or whether integration of sensory input is skewed is not known, but cognitive performance will decline. Understanding the neurobiology behind cognitive performance and potential methods to improve sustained attention could noticeably impact decision-making and ultimately save lives.
Neuroanatomy of Attention
It is generally accepted that alertness, focus, distractibility and impulse control are directly affected by sleep/wake cycles and circadian rhythms and are diffusely controlled by multiple areas of the brain. Prolonged wakefulness or short-term sleep deprivation deteriorates focus and produces widespread decreases in brain activity, particularly in the cortico-thalamic network that mediates attention and executive functioning. [6] Precisely how they are integrated neurologically is an active area of research. Numerous investigators have converged on initial theories of the neural pathways that control these functions by studying Parkinson’s disease, Attention Deficit Hyperactivity Disorder (ADHD) or brain injuries that produce similar symptoms. A. De La Fuente provides an overview of the relevant literature. [8] Most attentional and executive functions are believed to involve the dorsolateral and ventrolateral prefrontal cortex, dorsal anterior cingulate cortex, the caudate, cerebellum and the parietal cortex. [9]
Neuroanatomists have also been investigating the role of the basal ganglia with regard to attention and executive function. [10,11,12] The basal ganglia comprise an intricate neural network beneath the cortex. These nuclei accept inputs from multiple cortical areas and, through excitatory and inhibitory control loops, determine the appropriate response by adaptively controlling behavior through interactions with sensorimotor, motivational and cognitive areas. [13] Figure 1 shows a simplified version of the control loop. [14] Green arrows represent excitatory projections (glutamate) and red arrows represent inhibitory pathways (gamma-Aminobutyric acid (GABA)). The function of the basal ganglia is often described in terms of a “brake” theory or inhibition/ disinhibition. To sit still, the brain must inhibit/brake all movements except those that maintain posture. To move, the brain releases the brake (disinhibits), allowing voluntary movement. A key modulator of this circuit is dopamine, which is produced in the substantia nigra, represented by the blue arrow. Decreased production of dopamine, as seen in Parkinson’s disease and suggested in ADHD, causes perturbation of the excitatory/direct pathway resulting in motor movements as the circuit is no longer properly inhibited. Supporting this theory, volumetric differences in the striatum of children with ADHD have been reported, which may reduce its ability to inhibit the circuit properly. [15]
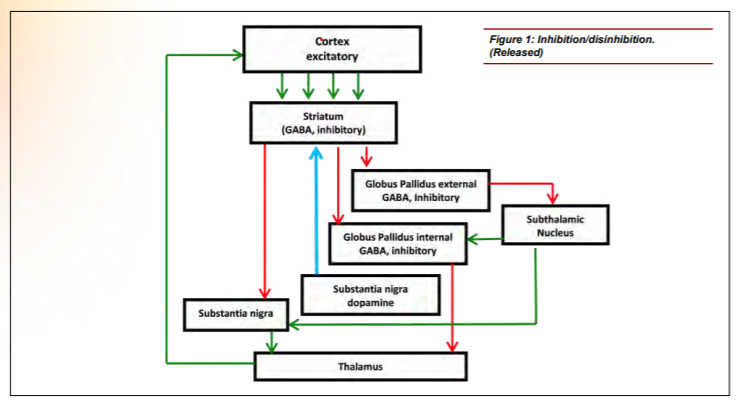
Figure 1: Inhibition/disinhibitin. (Released)
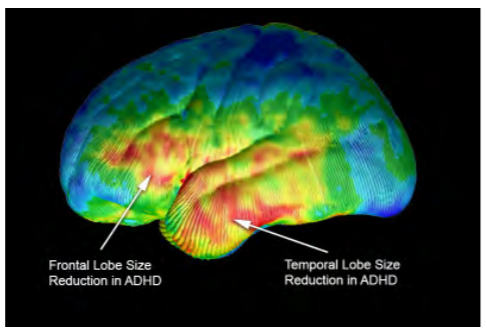
Figure 2: Volumetric differences. (Courtesy of Arthur W. Toga, Ph.D., University of Southern California Laboratory of Neuro Imaging, USC Mark and Mary Stevens Neuroimaging and Informatics Institute – www.loni.usc.edu/Released)
Current Methods to Improve Cognitive Performance
Numerous methods are used to reduce fatigue and enhance cognitive performance, including medications typically prescribed for ADHD. Common medications for ADHD are psychostimulants, such as methylphenidate. These stimulants interfere with a dopamine-reuptake mechanism, leaving more dopamine in the synapse to bind to the receiving cell. This compensates for a proposed deficiency of dopamine production in those with ADHD, resulting in a reduction of motor movements (fidgeting), increased concentration and less anxiety. However, when no dopamine deficiency is present, these medications can produce the opposite effect, leading to excitability, stress, distractibility and lack of focus. Additionally, these medications must be metabolized, which places stress on the liver. These controlled substances also pose a high risk for abuse, which can lead to dependence, cardiac risks and other health hazards. [16]
Caffeine, a central nervous system stimulant, is another method used to improve cognitive performance. During prolonged wakefulness, adenosine naturally accumulates in the brain, promotes drowsiness and lowers blood pressure as it binds to A2 a receptors in the basal ganglia. Caffeine prevents drowsiness by non-selectively blocking adenosine from binding to these receptors. [17] The A2 a receptor and dopamine D2 receptor have reciprocal antagonistic interactions in GABAergic neurons that drive the above-described indirect pathway. This is consistent with animal data that showed that A2a knockout mice had higher anxiety, aggression and blood pressure. [18] Continued use/overuse of caffeine has serious negative effects including aggression, [19] sleep disturbances, withdrawal headaches, cyclical alert/sleepy behavior, heart rate variability and increased blood pressure. [20]
As ingestible methods are known to cause the negative systemic effects discussed above, non-ingestible solutions are being investigated. Exercise is being more formally studied to identify its neurobiological effect on cognition. Animal models of rats with ADHD show hyperactivity, spatial learning memory deficit, reduction of tyrosine hydrox
ylase (TH) in the striatum and substantia nigra pars compacta and brain-derived neurotrophic factor (BDNF) in the hippocampus. Both treadmill exercise and methylphenidate alleviated the ADHD-induced hyperactivity and spatial learning memory impairment, increasing both TH and BDNF. [21] Exercise in rats also increased dopamine, serotonin and norepinephrine if fatigue was not reached. [22] In human studies, acute exercise significantly improved continuous-performance scores including response time, impulsivity and vigilance measures. [23]
More recently, two types of neurostimulation have been investigated to identify the effects on cognitive performance. The first is transcranial stimulation, which delivers either electrical or magnetic energy to the scalp. Based on questionnaires, mild improvement of attention scores was reported with no effect on anxiety or hyperactivity. [24] Extracellular dopamine release with repeated magnetic stimulation over the left prefrontal cortex has also been indicated. [25] This technology may prove to be very important in identifying specific neural pathways. However, the therapeutic benefit, practicality and safety have yet to be determined. [26]
The second type of neurostimulation, presented below, is a novel method of providing a safer form of stimulation through a wearable device without delivering electrical or magnetic stimulation. This is accomplished through frequency-specific vibratory bone conduction.
Feasibility of Novel Vibratory Technology
Several disparate bodies of work suggest that the vestibulocochlear nerve may play a key role in cognitive performance. Volumetric deficiencies and hypoperfusion in the prefrontal cortex and temporal lobe have been reported in children with ADHD. [27,28] Poor perfusion in these regions was also reported in drowsy versus non-drowsy individuals. [29] Both children and adults with attention deficit sleep disorders, autism and anxiety will self-soothe by body rocking, head shaking, bouncing or spinning — all of which naturally stimulate the vestibulocochlear nerve. Exercise stimulates this nerve and has been shown in animals and humans to increase perfusion, slow deterioration of volumetric reductions, [30] increase volume in the prefrontal cortex and temporal lobe and improve synaptic plasticity. [31] Given that this nerve originates in the pons, is tightly connected to the cerebellum and is easily accessible (shown below), we sought to artificially stimulate this region to identify its effect on attention, cerebral blood flow and cognitive performance.
Our first subject was a 29-year-old female with ADHD who was studied using quantitative electroencephalography (qEEG) at a licensed neurofeedback facility. The qEEG is conducted by instrumenting the subject with a standard 10-20 EEG electrode cap. Baseline data were collected at rest and after mechanically stimulating over the right mastoid unilaterally. The qEEG system automatically collects data and compares it to a normative database of age and gender matched controls. Data are then automatically scored as being consistent with either the ADHD or “normal” population. A representative image of the study is shown in Figure 3. The top panel shows a characteristically familiar profile of an individual with ADHD. Broad-spread hypoperfusion is noticeable in several areas of the cortex. The bottom panel illustrates that after five minutes of use, many of these areas scored similarly to individuals without ADHD. These data show promise, as they are very similar to other published effects of medication, but do not negatively impact the heart or liver. While the duration of this effect is not yet known, we believe it is worth studying both the effect of wearing the device longer and the overall long-lasting effect.
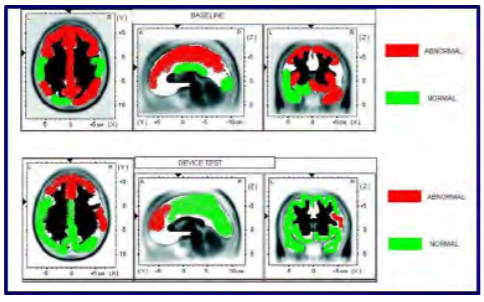
Figure 3: Cerebral perfusion. (Released)
Next, five healthy college students were studied using qEEG. Individuals were instrumented as above. Resting EEG data were collected for one minute before and after using the stimulation for five minutes on either the right or left mastoid. qEEG data were separated into frequency bands for each lead. The theta/beta ratios for the CZ location, as well as F3 and F4 locations, have been reported as viable objective indicators of attention. [32,33] A decreased ratio indicates better focus. A meta-analysis of qEEG data supports that the theta/beta ratio increases with attention deficit. [34] The summary data is presented in Figure 4, which shows that the stimulation decreased the theta/beta ratio in almost every case, suggesting an increase in focus/alertness. The consistency across leads and across subjects supported further research into identifying cognitive benefits of vestibulocochlear stimulation.
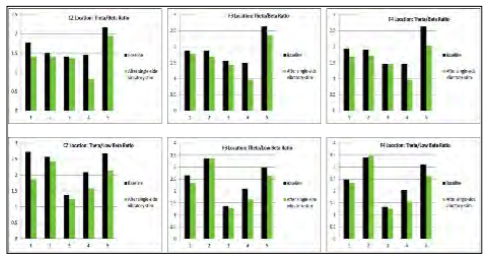
Figure 4: Summary of qEEG data. (Released)
To ascertain if vestibulocochlear stimulation has any effect on cognitive performance, a wearable device was designed that delivers vibratory stimulation bilaterally in a specified pattern. Ten volunteers with self-reported distractibility or attention deficit participated. The Test of Variables of Attention (TOVA) was used to provide objective measures, including errors of commission, errors of omission and response time variability. Each test took approximately 20 minutes and required the user to click a button when they either saw or heard a designated target. Each volunteer was tested before and after wearing the device for 30 minutes. The software presents the targets in random order to minimize any potential learning effect. All data were collected automatically and independently scored by the TOVA Company, which compared each subject’s data to a database of age and gender matched controls. The results indicate whether each subject’s scores align with either an ADHD or normal population. The test is comprised of two 10-minute segments. The first half tests one’s ability to stay focused when the target is presented infrequently. (Does the subject daydream or overcompensate?) The second half tests performance when the target is presented frequently. (Does the subject get anxious or anticipate responses?) In total, 20 segments were collected as shown in Figure 5 (10 subjects; slower segment and faster segment). Green indicates the test result aligned with the normal data for that subject’s age and gender. Red indicates the test result aligned with the ADHD data.
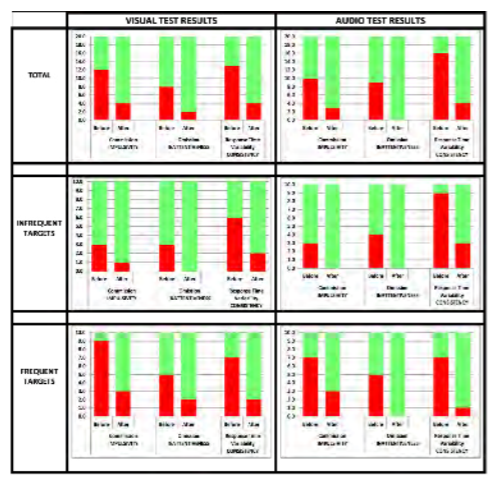
Figure 5: Continuous Performance Testdata summary. (Released)
Observations
Across the data set, the total number of errors of commission (impulsivity), omission (inattentiveness) and response time variability (consistency) improved after wearing the device. This particular population of 10 volunteers had more difficulty with errors of commission (impulsivity) and inconsistent reaction time. Errors of omission were particularly noticeable in the more rapid environment. Errors of omission and reaction time variability occurred slightly more frequently during the auditory test than the visual test, and the subjects responded well to the stimulation. Equally important, none of the volunteers became drowsy, nauseated or anxious.
While each of the tests within the third study has shown promising results, due to the small population size, no definitive conclusions can be drawn. The TOVA test also had several limitations. Firstly, the comparison database for the auditory database is not segmented adults. Secondly, the testing environment separates the visual test and the auditory test. This helps isolate potential issues but does not challenge the subject with both auditory and visual information simultaneously. Finally, if the data falls into the “normal” range, the data cannot be segmented further to see if there is improvement after using a particular intervention.
Discussion
While the exact mechanism of action is not yet known, there are clues to the anatomy involved. E. D’Angelo and S. Casali propose that the cerebellum acts as a co-processor that integrates sensory inputs, memory, emotion and timing information through various feedback loops, and then affects cognition and motor control. [35] This notion is supported by the multiple vestibulocochlear neuropathways developed for different functions. It is known that the cerebellum tightly controls the oculomotor activity and is directly connected to the vestibulo-ocular reflex and that vestibular inputs to the thalamus communicate with the motor cortex for motor control. More recently, M. Hitier, S. Besnard and P. F. Smith describe five currently known and proposed neuropathways between the cerebellum and the cortex. [36] Of particular interest are the proposed direct connections to the cerebellum and to the striatum. Referring to the previous discussion of the basal ganglia, the circuit is presented again in Figure 6 with these connections added. If these pathways exist, it may describe a direct role in modulating the circuit. Instead of effecting dopamine, it most likely would have an inhibitory influence (purple arrow to the striatum). This might explain the reduction of errors of commission (excitability) and more consistent reaction time seen in our feasibility studies. It is not yet known how this stimulation affects the elegant control experienced by the brain, but at a conceptual level, it could be that keeping this circuit active at an optimal frequency, ideally below a level that would cause gross motor movement, keeps the cerebellum, visual system, auditory system and executive function engaged and properly inhibits the thalamus.
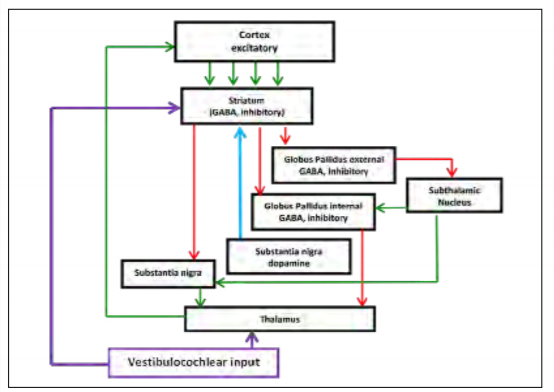
Figure 6: Proposed feedback loop. (Released)
Current and Future Studies
Due to the limitations of the test environment previously used, a more robust test that intermixes audio and visual targets simultaneously has been chosen for the current study. This tests one’s ability to rapidly switch focus and make a decision. It also allows differentiation for individuals that are higher functioning in the “normal” range. Future studies of interest include testing individuals on a longitudinal basis; testing individuals with Post-Traumatic Stress Disorder (PTSD); and, due to the perfusion data, testing the healing time of head injuries, such as concussion or stroke.
Potential DoD Applications
Further study could utilize this EEG technique to evaluate individuals with PTSD, concussion or stroke. This research could prove beneficial to the DoD as these injuries are common among service members across all military branches. Study results could lead to recommendations that could help mitigate and prevent these warfighter injuries. Based on the performance data, the most likely follow-on studies to the second study could be centered on decision-making and focus in a rapid manner without getting overwhelmed. Drone pilots would be a fitting group for a follow-on study, as their responsibilities require quick decisions and intense focus.
References
1. Russo, M., Fiedler, E., Thomas, M., & McGhee, J. (2005). Cognitive performance in operational environments. In Strategies to maintain combat readiness during extended deployments – A human systems approach (pp. 14-1-14-16). Meeting Proceedings RTO-MP-HFM-124, Paper 14. Neuilly-surSeine, France: RTO.
2. Caldwell, J. L., Chandler, J. F., & Hartzler, B. M. (2012). Battling fatigue in aviation: Recent advancements in research and practice. Journal of Medical Sciences, 32(2), 47-56.
3. Army Research Laboratory. (2014, April). Army Research Laboratory Technical Strategy. Retrieved from https://www.arl.army.mil/www/pages/172/docs/ARL_Technical_Strategy_FINAL.pdf (accessed May 9, 2017).
4. Wright-Patterson Air Force Base. (2017, March 28). AFOSR – Information and Networks. Retrieved from http://www.wpafb.af.mil/Welcome/Fact-Sheets/Display/Article/842033/ (accessed May 9, 2017).
5. U.S. Naval Research Laboratory. (n.d.). Warfighter Human System Integration Laboratory (WHSIL). Retrieved from https://www.nrl.navy.mil/itd/imda/research/5582/WHSIL (accessed May 9, 2017).
6. Rouch, I., Wild, P., Ansiau, D., & Marquié, J.-C. (2005). Shiftwork experience, age and cognitive performance. Ergonomics, 48(10), 1282-1293. doi:10.1080/00140130500241670
7. Kazemi, R, Haidarimoghadam, R., Motamedzadeh, M., Golmohamadi, Soltanian, A., & Zoghipaydar, M. R. (2016). Effects of Shift Work on Cognitive Performance, Sleep Quality, and Sleepiness among Petrochemical Control Room Operators. Journal of Circadian Rhythms, 14(1), 1–8. doi: 10.5334/
jcr.134
8. De La Fuente, A., Xia, S., Branch C., & Li, X. (2013) A review of attention-deficit/hyperactivity disorder from the perspective of brain networks. Frontiers in Human Neuroscience, 7:192, 1-6. doi: 10.3389/fnhum.2013.00192
9. Bush, G. (2010). Attention-Deficit/Hyperactivity Disorder and Attention Networks. Neuropsychopharmacology, 35, 278–300. doi:10.1038.npp.2009.120
10. Circuits within the Basal Ganglia System. (2001) In D. Purves, G. J. Augustine, D. Fitzpatrick, L. C. Katz, A.-S. La Mantia, J. O. McNamara, & S. M. Williams (Eds.), Neuroscience (2nd ed.) Sunderland, MA: Sinauer Associates.
11. Knierim, J. (n.d.). Basal Ganglia [Electronic Textbook]. Retrieved from http://neuroscience.uth.tmc.edu/s3/chapter04.html (accessed May 9, 2017).
12. D’Angelo, E., Casali, S. (2013). Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Frontiers in Neural Circuits, 6:116, 1-23. doi: 10.3389/fncir.2012.00116
13. Graybiel, A. M., Aosaki, T., & Kimura, M. (1994). The basal ganglia and adaptive motor control. Science, 365(5180), 1826–1831. doi: 10.1126/science.8091209
14. Freeze, B. S., Kravitz, A. V., Hammack, N., Berke, J. D., & Kreitzer, A. C. (2013). Control of Basal Ganglia Output by Direct and Indirect Pathway Projection Neurons. The Journal of Neuroscience, 33(47), 18531-18539. doi: 10.1523/JNEUROSCI.1278.13.2013
15. Hynd, G.W., Hern, K. L., Novey, E. S., Eliopulos, D., Marshall, R., Gonzales, J. J., & Voeller, K. K. (1993). Attention Deficit-Hyperactivity Disorder and Asymmetry of the Caudate Nucleus. Journal of Child Neurology, 8(4), 339-347.
16. Berman, S. M., Kuczenski, R., McCracken, J. T., & London, E. D. (2009). Potential adverse effects of amphetamine treatment on brain and behavior: a review. Molecular Psychiatry, 14(2), 123-142. doi: 10.1038/mp.2008.90
17. Kozial, L. F. & Budding, D. E. (2009). Subcortical structures and cognition: Implications for neuropsychological assessment. (p. 405). New York, NY: Springer.
18. Ledent, C, Vaugeois, J.-M., Schiffman, S. N., Pedrazzini, T., El Yacoubi, M., Vanderhaeghen, J.-J., … & Parmentier, M. (1997). Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature, 388(6643), 674-678.
19. Martin, C. A., Cook C., Woodring, J. H., Burkhardt, G., Guenthner, G., Omar, H. A., & Kelly, T. H. (2008). Caffeine Use: Association with Nicotine Use, Aggression, and Other Psychopathology in Psychiatric and Pediatric Outpatient Adolescents. The Scientific World Journal, 8, 512–516. doi:
10.1100/tsw.2008.82
20. Koenig J., Jarczok, M. N, Kuhn, W., Morsch, K., Schäfer, A., Hillecke, T. K., & Thayer, J. F. (2013). Impact of Caffeine on Heart Rate Variability: A Systematic Review. Journal of Caffeine Research, 3(1), 22-37. doi:10.1089/jcr.2013.0009
21. Kim H., Heo, H.-I., Kim, D.-H., Ko, I.-G., Lee, S.-S., Kim, S.-E., … & Kim, C.-J., (2013). Treadmill exercise and methylphenidate ameliorate symptoms of attention deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neuroscience Letters, 504(1), 35-9. doi: 10.1016/j.
neulet.2011.08.052
22. Bailey, S. P., Davis, J. M., & Ahlborn, E. N. (1993). Neuroendocrine and substrate responses to altered brain 5-HT activity during prolonged exercise to fatigue. Journal of Applied Physiology, 74(6), 3006–3012.
23. Medina, J. A., Netto, T. L., Muszkat, M., Medina, A. C., Botter, D., Orbetelli, R., … & Miranda, M. C. (2010). Exercise impact on sustained attention of ADHD children, methylphenidate effects. ADHD Attention Deficit and Hyperactivity Disorders, 2(1), 49-58. doi: 10.1007/s12402-009-0018-y
24. Bloch, Y., Harel, E. V., Aviram, S., Govezensky, J., Ratzoni, G., & Levkovitz, Y. (2010). Positive effects of repetitive transcranial magnetic stimulation on attention in ADHD Subjects: A randomized controlled pilot study. The World Journal of Biological Psychiatry 11(5), 755-758. doi:
10.3109/15622975.2010.484466
25. Strafella, A. P., Paus, T., Barrett, J., & Dagher A. (2001). Repetitive Transcranial Magnetic Stimulation of the Human Prefrontal Cortex Induces Dopamine Release in the Caudate Nucleus. Journal of Neuroscience, 21(15): RC157, 1-4.
26. Thomas, M., Sing, H., Belenky, G., Holcomb, H., Mayberg, H., Dannals, R., … & Redmond, D. (2000). Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of
24 h of sleep deprivation on waking human regional brain activity. Journal of Sleep Research, 9(4), 335–352. doi: 10.1046/j.1365-2869.2000.00225.x
27. Bower, B. (2003, Nov. 29). ADHD’s Brain Trail: Cerebral clues emerge for attention disorder. Science News, 164(22), 339.
28. Kaya, G. C., Pekcanlar, A., Bekis, R., Ada, E., Miral, S., Emiroğlu, N., & Durak, H. (2002). Technetium-99m HMPAO brain SPECT in children with attention deficit hyperactivity disorder. Annals of Nuclear Medicine, 16(8) 527-31. doi: 10.1007/BF02988629
29. Poudel, G. R., Innes, C. R., & Jones, R. D. (2012). Cerebral Perfusion Differences Between Drowsy and Nondrowsy Individuals After Acute Sleep Restriction. Sleep, 35(8), 1085-1096. doi: 10.5665/sleep.1994
30. Radiological Society of North America. (2016). Aerobic exercise preserves brain volume and improves cognitive function. [Press Release]. Retrieved from https://press.rsna.org/timssnet/Media/pressreleases/14_pr_target.cfm?id=1921 (accessed May 9, 2017).
31. Colcombe, S. J., Kramer, A. F., Erickson, K. I., Scalf, P., McAuley, E., Cohen, N. J., …& Elavsky, S. (2004) Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences, 101(9), 3316–3321. doi: 10.1073/pnas.0400266101
32. Amer, D. A., Rakhawy, M. Y., & El Kholy, S. H. (2010). Quantitative EEG in Children with Attention Deficit Hyperactivity Disorder. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery, 47(3), 399-406.
33. Sangal, R. B. & Sangal, J.M. (2014). Use of EEG Beta-1 Power and Theta/Beta Ratio Over Broca’s Area to confirm Diagnosis of Attention Deficit/Hyperactivity Disorder in Children. Clinical EEG and Neuroscience, 46(3), 177-82. doi: 10.1177/1550059414527284
34. Snyder, S. M. & Hall, J. R. (2006). A Meta-analysis of Quantitative EEG Power Associated With Attention-Deficit Hyperactivity Disorder. Journal of Clinical Neurophysiology, 23(5), 441-456. doi: 10.1097/01. wnp.0000221363.12503.78
35. D’Angelo, E. & Casali, S. (2013). Seeking a unified framework for cerebellar function
and dysfunction: from circuit operations to cognition. Frontiers in Neural Circuits, 6:116,
1-23. doi: 10.3389/fncir.2012.00116
36. Hitier, M., Besnard, S., & Smith, P. F. (2014). Vestibular pathways involved in cognition. Frontiers in Integrative Neuroscience, 8:59, 1-16. doi: 10.3389/fnint.2014.00059


