The Department of Defense operates worldwide, including in remote and austere environments without access to modern medical facilities. The task of diagnosing and caring for sick
and injured soldiers on the battlefield, especially in isolated regions, remains a challenge. The austere environment, coupled with the potential to contract local diseases, creates a need for rapid diagnostics and treatment. Developing in-field detection of pathogens, disease and biological warfare agents allows warfighters to request necessary medical evacuation or quarantine for sick or contagious troops; thus mitigating the risk of exposure to other personnel.
Soon, taking clinical and environmental samples to a laboratory for high quality, highly reliable molecular diagnostics tests will be outdated. The era of point of care and point of need diagnostics tests is here and the applications for these diagnostic tests are broad, diverse and limited only by the imagination. But, ensuring the sensitivity, specificity, robustness and reliability of performance of point of care and point of need molecular diagnostics is essential.
This means the platform or device running the test, as well as the assays, or the test itself, must be thoughtfully and rigorously designed, and have proven performance. Investment in this type of product represents a significant opportunity for DoD to improve the survivability rate for its soldiers and maximize the potential of field medics to treat as many ill and wounded as possible.
Researchers with the PositiveID Corporation designed Firefly Dx, a hand-held, battery powered, cartridge-based, rapid, multiplex, real-time polymerase chain reaction diagnostic device for point of care and point of need, lab-quality detection of pathogenic organisms, biomarkers and single nucleotide polymorphism identity assays.
The device will allow detection and identification of Influenza at the point of need with lab-grade results in 15 to 30 minutes. This is simply not possible with existing systems, which require lab-based equipment, technical personnel, and can take hours or even days to provide results. The ability to rapidly test and diagnose a contagious or deadly illness will allow medics to administer appropriate treatment for the correct illness instead of providing general care while they wait for lab results. In remote locations, the option to send for a laboratory test is not feasible. The use of the Firefly Dx would allow for testing and identification of an infectious disease when previously there was no option.
The Firefly Dx is a two-part device consisting of a portable hand-held instrument and disposable single-use cartridges. The cartridges will contain all of the reagents necessary for sample lysis, purification and PCR analysis of the extracted DNA or RNA. Firefly Dx will be able to process a variety of sample types, including whole blood, buccal and nasopharyngeal swabs, urine and
environmental field samples. PositiveID Corporation is teaming up with GenArraytion, Inc., to provide high quality, rapidly developed, proven assays.
GenArraytion brings its highly successful approach to assay development to bear on development of Influenza assays. GenArraytion’s MultiFLEXTM Bioassays for infectious disease agents provide a significant advance in multiplexing flexibility, with more than 20 DNA and/or RNA targets in a single assay panel that can be mixed and matched to suit various platforms and operational scenarios.
The multiplex PCR assays are readily configurable, highly specific and ready for use in real-time and bead-based, end-point platforms. The assays are available for clinical pathogens and veterinary disease agents as well as food or waterborne pathogens and biological threats. Recently the company developed panels for both tick and mosquito-borne pathogens including Aedes aegypti (yellow fever mosquito) associated viruses.
GenArraytion’s approach to molecular infectious disease diagnostic assay development leverages available genetic sequence information by mining the data for highly informative genetic
targets that are optimized and validated using a robust iterative laboratory assay development process. The company demonstrated the ability to rapidly develop high fidelity molecular infectious disease tests and various real-time PCR platforms, as well as more highly multiplexed assays, including planar microarrays and bead-based assays.
Figure 1 shows a graph of real-time PCR raw data generated in the Firefly Flu Dx integrated breadboard following RNA extraction and sequence specific reverse transcription, using GenArraytion Influenza assays. GenArraytion has performed a bioinformatics analysis of flu strains published in GenBank and we have designed a screening array containing over 15,000 sequences representing unique regions of various Influenza isolates. Assay targets were selected based on the specificity of more than 20 Influenza isolates.
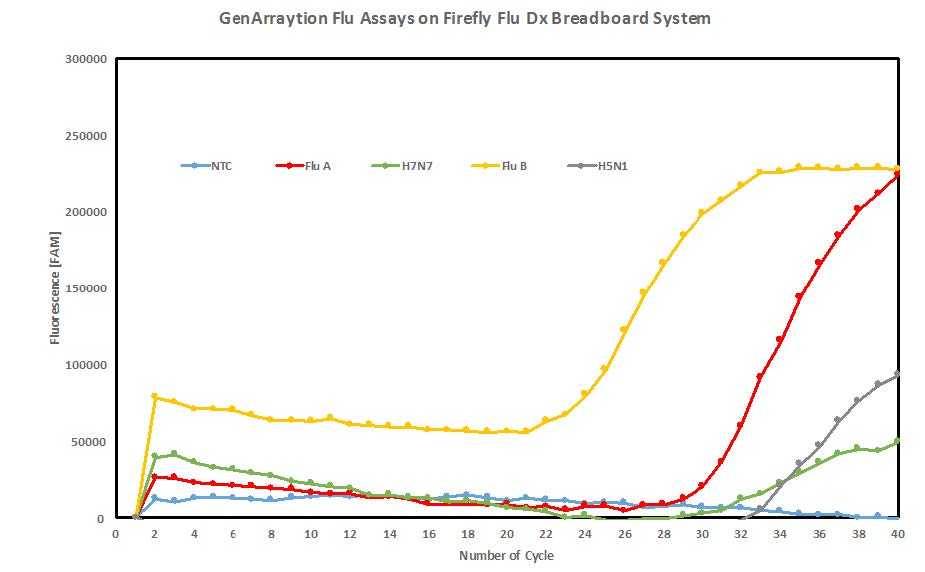
Figure 1. Real-time PCR raw data generated by the Firefly Flu Dx breadboard system following RNA extraction and sequence specific reverse transcription using GenArraytion Influenza assays. Target amounts were all 500 pg. (Released)


